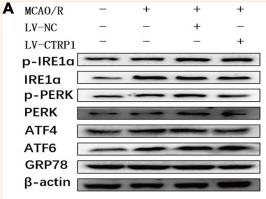
Phospho-IRE1 (Ser724) Antibody - #AF7150
| Product: | Phospho-IRE1 (Ser724) Antibody |
| Catalog: | AF7150 |
| Description: | Rabbit polyclonal antibody to Phospho-IRE1 (Ser724) |
| Application: | WB IHC IF/ICC |
| Cited expt.: | WB, IHC, IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 110kDa; 110kD(Calculated). |
| Uniprot: | O75460 |
| RRID: | AB_2843590 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF7150, RRID:AB_2843590.
Fold/Unfold
Endoplasmic reticulum (ER) to nucleus signalling 1; Endoplasmic reticulum to nucleus signaling 1; Endoplasmic reticulum-to-nucleus signaling 1; Endoribonuclease; ER to nucleus signaling 1; ERN 1; Ern1; ERN1_HUMAN; hIRE 1p; hIRE1p; Inositol requiring 1; Inositol requiring 1, S. cerevisiae, homolog of; Inositol requiring enzyme 1, S. cerevisiae, homolog of; Inositol requiring protein 1; inositol-requiring enzyme 1; Inositol-requiring protein 1; IRE 1; IRE 1a; IRE 1P; Ire1 alpha; Ire1-alpha; IRE1a; Ire1alpha; IRE1P; MGC163277; MGC163279; Protein kinase/endoribonuclease; RGD1559716; Serine/threonine protein kinase/endoribonuclease IRE1; Endoplasmic reticulum (ER) to nucleus signalling 1; Endoplasmic reticulum to nucleus signaling 1; Endoplasmic reticulum-to-nucleus signaling 1; Endoribonuclease; ER to nucleus signaling 1; ERN 1; Ern1; ERN1_HUMAN; hIRE 1p; hIRE1p; Inositol requiring 1; Inositol requiring 1, S. cerevisiae, homolog of; Inositol requiring enzyme 1, S. cerevisiae, homolog of; Inositol requiring protein 1; inositol-requiring enzyme 1; Inositol-requiring protein 1; IRE 1; IRE 1a; IRE 1P; Ire1 alpha; Ire1-alpha; IRE1a; Ire1alpha; IRE1P; MGC163277; MGC163279; Protein kinase/endoribonuclease; RGD1559716; Serine/threonine protein kinase/endoribonuclease IRE1;
Immunogens
A synthesized peptide derived from human IRE1 around the phosphorylation site of phospho S724.
- O75460 ERN1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MPARRLLLLLTLLLPGLGIFGSTSTVTLPETLLFVSTLDGSLHAVSKRTGSIKWTLKEDPVLQVPTHVEEPAFLPDPNDGSLYTLGSKNNEGLTKLPFTIPELVQASPCRSSDGILYMGKKQDIWYVIDLLTGEKQQTLSSAFADSLCPSTSLLYLGRTEYTITMYDTKTRELRWNATYFDYAASLPEDDVDYKMSHFVSNGDGLVVTVDSESGDVLWIQNYASPVVAFYVWQREGLRKVMHINVAVETLRYLTFMSGEVGRITKWKYPFPKETEAKSKLTPTLYVGKYSTSLYASPSMVHEGVAVVPRGSTLPLLEGPQTDGVTIGDKGECVITPSTDVKFDPGLKSKNKLNYLRNYWLLIGHHETPLSASTKMLERFPNNLPKHRENVIPADSEKKSFEEVINLVDQTSENAPTTVSRDVEEKPAHAPARPEAPVDSMLKDMATIILSTFLLIGWVAFIITYPLSMHQQQQLQHQQFQKELEKIQLLQQQQQQLPFHPPGDTAQDGELLDTSGPYSESSGTSSPSTSPRASNHSLCSGSSASKAGSSPSLEQDDGDEETSVVIVGKISFCPKDVLGHGAEGTIVYRGMFDNRDVAVKRILPECFSFADREVQLLRESDEHPNVIRYFCTEKDRQFQYIAIELCAATLQEYVEQKDFAHLGLEPITLLQQTTSGLAHLHSLNIVHRDLKPHNILISMPNAHGKIKAMISDFGLCKKLAVGRHSFSRRSGVPGTEGWIAPEMLSEDCKENPTYTVDIFSAGCVFYYVISEGSHPFGKSLQRQANILLGACSLDCLHPEKHEDVIARELIEKMIAMDPQKRPSAKHVLKHPFFWSLEKQLQFFQDVSDRIEKESLDGPIVKQLERGGRAVVKMDWRENITVPLQTDLRKFRTYKGGSVRDLLRAMRNKKHHYRELPAEVRETLGSLPDDFVCYFTSRFPHLLAHTYRAMELCSHERLFQPYYFHEPPEPQPPVTPDAL
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Serine/threonine-protein kinase and endoribonuclease that acts as a key sensor for the endoplasmic reticulum unfolded protein response (UPR). In unstressed cells, the endoplasmic reticulum luminal domain is maintained in its inactive monomeric state by binding to the endoplasmic reticulum chaperone HSPA5/BiP. Accumulation of misfolded protein in the endoplasmic reticulum causes release of HSPA5/BiP, allowing the luminal domain to homodimerize, promoting autophosphorylation of the kinase domain and subsequent activation of the endoribonuclease activity. The endoribonuclease activity is specific for XBP1 mRNA and excises 26 nucleotides from XBP1 mRNA. The resulting spliced transcript of XBP1 encodes a transcriptional activator protein that up-regulates expression of UPR target genes. Acts as an upstream signal for ER stress-induced GORASP2-mediated unconventional (ER/Golgi-independent) trafficking of CFTR to cell membrane by modulating the expression and localization of SEC16A.
Autophosphorylated following homodimerization. Autophosphorylation promotes activation of the endoribonuclease domain.
ADP-ribosylated by PARP16 upon ER stress, which increases both kinase and endonuclease activities.
Endoplasmic reticulum membrane>Single-pass type I membrane protein.
Ubiquitously expressed. High levels observed in pancreatic tissue.
Belongs to the protein kinase superfamily. Ser/Thr protein kinase family.
Research Fields
· Cellular Processes > Transport and catabolism > Autophagy - animal. (View pathway)
· Cellular Processes > Cell growth and death > Apoptosis. (View pathway)
· Genetic Information Processing > Folding, sorting and degradation > Protein processing in endoplasmic reticulum. (View pathway)
· Human Diseases > Endocrine and metabolic diseases > Non-alcoholic fatty liver disease (NAFLD).
· Human Diseases > Neurodegenerative diseases > Alzheimer's disease.
References
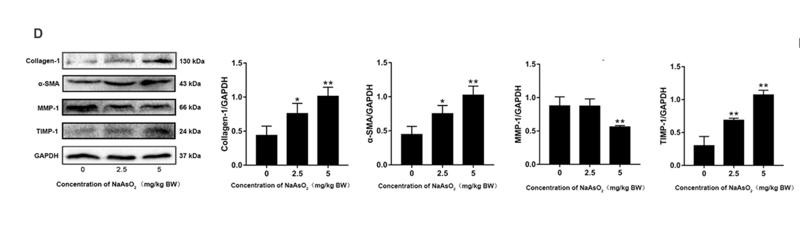
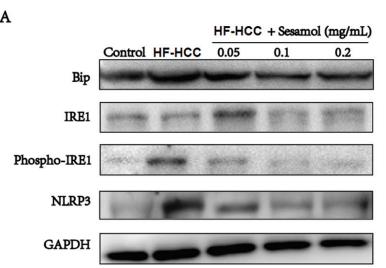
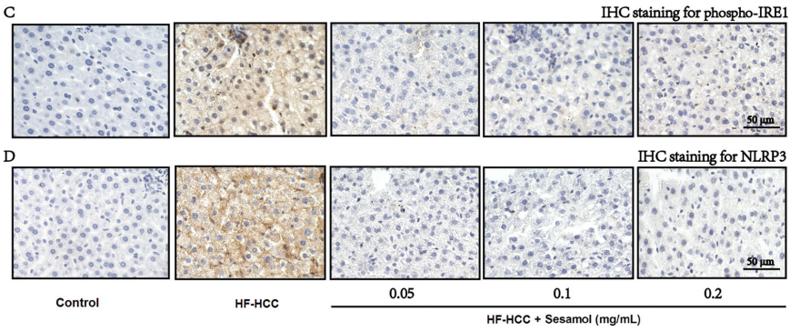
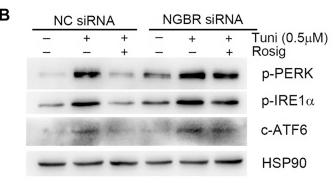
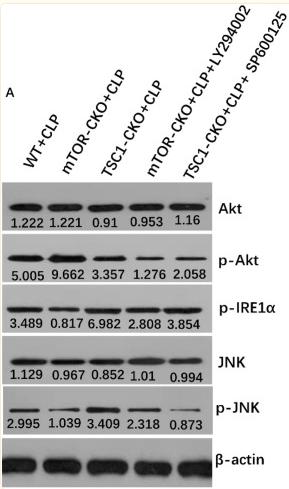
Application: WB Species: human Sample: Molm13 cell
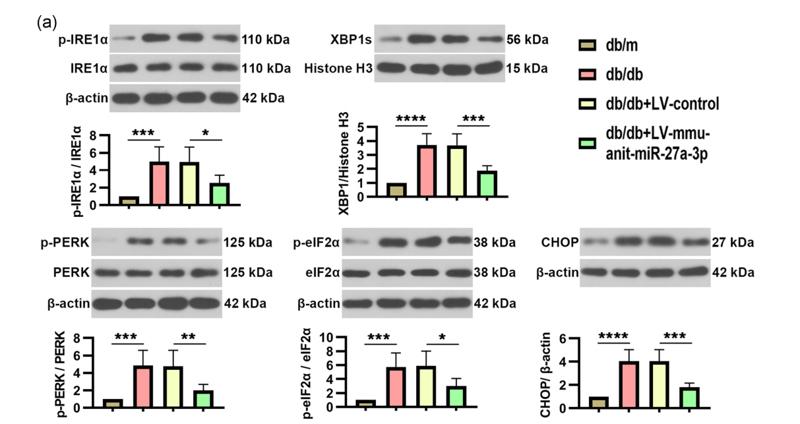
Application: WB Species: Human Sample: LN229 and U251 cells
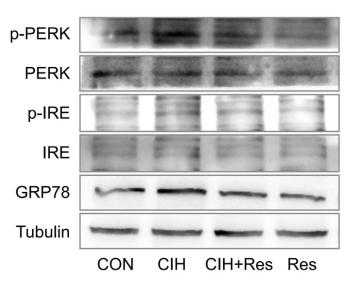
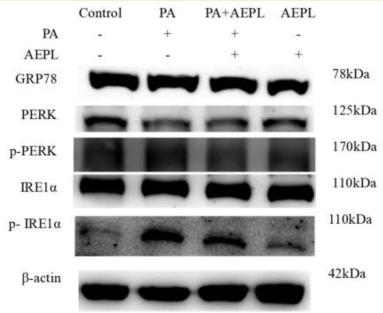
Application: WB Species: Human Sample: HepG2 Cells
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.