Phospho-Smad3 (Ser425) Antibody - #AF3362
| Product: | Phospho-Smad3 (Ser425) Antibody |
| Catalog: | AF3362 |
| Description: | Rabbit polyclonal antibody to Phospho-Smad3 (Ser425) |
| Application: | WB IHC IF/ICC |
| Cited expt.: | WB, IHC, IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 58kDa; 48kD(Calculated). |
| Uniprot: | P84022 |
| RRID: | AB_2834777 |
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF3362, RRID:AB_2834777.
Fold/Unfold
DKFZP586N0721; DKFZp686J10186; hMAD 3; hMAD-3; hSMAD3; HSPC193; HST17436; JV15 2; JV15-2; JV152; LDS1C; LDS3; MAD (mothers against decapentaplegic Drosophila) homolog 3; MAD homolog 3; Mad homolog JV15 2; Mad protein homolog; MAD, mothers against decapentaplegic homolog 3; Mad3; MADH 3; MADH3; MGC60396; Mothers against decapentaplegic homolog 3; Mothers against DPP homolog 3; SMA and MAD related protein 3; SMAD 3; SMAD; SMAD family member 3; SMAD, mothers against DPP homolog 3; Smad3; SMAD3_HUMAN;
Immunogens
A synthesized peptide derived from human Smad3 around the phosphorylation site of Ser425.
- P84022 SMAD3_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MSSILPFTPPIVKRLLGWKKGEQNGQEEKWCEKAVKSLVKKLKKTGQLDELEKAITTQNVNTKCITIPRSLDGRLQVSHRKGLPHVIYCRLWRWPDLHSHHELRAMELCEFAFNMKKDEVCVNPYHYQRVETPVLPPVLVPRHTEIPAEFPPLDDYSHSIPENTNFPAGIEPQSNIPETPPPGYLSEDGETSDHQMNHSMDAGSPNLSPNPMSPAHNNLDLQPVTYCEPAFWCSISYYELNQRVGETFHASQPSMTVDGFTDPSNSERFCLGLLSNVNRNAAVELTRRHIGRGVRLYYIGGEVFAECLSDSAIFVQSPNCNQRYGWHPATVCKIPPGCNLKIFNNQEFAALLAQSVNQGFEAVYQLTRMCTIRMSFVKGWGAEYRRQTVTSTPCWIELHLNGPLQWLDKVLTQMGSPSIRCSSVS
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Receptor-regulated SMAD (R-SMAD) that is an intracellular signal transducer and transcriptional modulator activated by TGF-beta (transforming growth factor) and activin type 1 receptor kinases. Binds the TRE element in the promoter region of many genes that are regulated by TGF-beta and, on formation of the SMAD3/SMAD4 complex, activates transcription. Also can form a SMAD3/SMAD4/JUN/FOS complex at the AP-1/SMAD site to regulate TGF-beta-mediated transcription. Has an inhibitory effect on wound healing probably by modulating both growth and migration of primary keratinocytes and by altering the TGF-mediated chemotaxis of monocytes. This effect on wound healing appears to be hormone-sensitive. Regulator of chondrogenesis and osteogenesis and inhibits early healing of bone fractures. Positively regulates PDPK1 kinase activity by stimulating its dissociation from the 14-3-3 protein YWHAQ which acts as a negative regulator.
Phosphorylated on serine and threonine residues. Enhanced phosphorylation in the linker region on Thr-179, Ser-204 and Ser-208 on EGF and TGF-beta treatment. Ser-208 is the main site of MAPK-mediated phosphorylation. CDK-mediated phosphorylation occurs in a cell-cycle dependent manner and inhibits both the transcriptional activity and antiproliferative functions of SMAD3. This phosphorylation is inhibited by flavopiridol. Maximum phosphorylation at the G(1)/S junction. Also phosphorylated on serine residues in the C-terminal SXS motif by TGFBR1 and ACVR1. TGFBR1-mediated phosphorylation at these C-terminal sites is required for interaction with SMAD4, nuclear location and transactivational activity, and appears to be a prerequisite for the TGF-beta mediated phosphorylation in the linker region. Dephosphorylated in the C-terminal SXS motif by PPM1A. This dephosphorylation disrupts the interaction with SMAD4, promotes nuclear export and terminates TGF-beta-mediated signaling. Phosphorylation at Ser-418 by CSNK1G2/CK1 promotes ligand-dependent ubiquitination and subsequent proteasome degradation, thus inhibiting SMAD3-mediated TGF-beta responses. Phosphorylated by PDPK1.
Acetylation in the nucleus by EP300 in the MH2 domain regulates positively its transcriptional activity and is enhanced by TGF-beta.
Poly-ADP-ribosylated by PARP1 and PARP2. ADP-ribosylation negatively regulates SMAD3 transcriptional responses during the course of TGF-beta signaling.
Ubiquitinated. Monoubiquitinated, leading to prevent DNA-binding. Deubiquitination by USP15 alleviates inhibition and promotes activation of TGF-beta target genes. Ubiquitinated by RNF111, leading to its degradation: only SMAD3 proteins that are 'in use' are targeted by RNF111, RNF111 playing a key role in activating SMAD3 and regulating its turnover (By similarity). Undergoes STUB1-mediated ubiquitination and degradation.
Cytoplasm. Nucleus.
Note: Cytoplasmic and nuclear in the absence of TGF-beta. On TGF-beta stimulation, migrates to the nucleus when complexed with SMAD4 (PubMed:15799969). Through the action of the phosphatase PPM1A, released from the SMAD2/SMAD4 complex, and exported out of the nucleus by interaction with RANBP1 (PubMed:16751101, PubMed:19289081). Co-localizes with LEMD3 at the nucleus inner membrane (PubMed:15601644). MAPK-mediated phosphorylation appears to have no effect on nuclear import (PubMed:19218245). PDPK1 prevents its nuclear translocation in response to TGF-beta (PubMed:17327236).
The MH1 domain is required for DNA binding. Also binds zinc ions which are necessary for the DNA binding.
The MH2 domain is required for both homomeric and heteromeric interactions and for transcriptional regulation. Sufficient for nuclear import.
The linker region is required for the TGFbeta-mediated transcriptional activity and acts synergistically with the MH2 domain.
Belongs to the dwarfin/SMAD family.
Research Fields
· Cellular Processes > Cell growth and death > Cell cycle. (View pathway)
· Cellular Processes > Transport and catabolism > Endocytosis. (View pathway)
· Cellular Processes > Cell growth and death > Cellular senescence. (View pathway)
· Cellular Processes > Cellular community - eukaryotes > Adherens junction. (View pathway)
· Cellular Processes > Cellular community - eukaryotes > Signaling pathways regulating pluripotency of stem cells. (View pathway)
· Environmental Information Processing > Signal transduction > FoxO signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > Wnt signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > TGF-beta signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > Apelin signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > Hippo signaling pathway. (View pathway)
· Human Diseases > Infectious diseases: Parasitic > Chagas disease (American trypanosomiasis).
· Human Diseases > Infectious diseases: Viral > Hepatitis B.
· Human Diseases > Infectious diseases: Viral > HTLV-I infection.
· Human Diseases > Cancers: Overview > Pathways in cancer. (View pathway)
· Human Diseases > Cancers: Specific types > Colorectal cancer. (View pathway)
· Human Diseases > Cancers: Specific types > Pancreatic cancer. (View pathway)
· Human Diseases > Cancers: Specific types > Chronic myeloid leukemia. (View pathway)
· Human Diseases > Cancers: Specific types > Hepatocellular carcinoma. (View pathway)
· Human Diseases > Cancers: Specific types > Gastric cancer. (View pathway)
· Human Diseases > Immune diseases > Inflammatory bowel disease (IBD).
· Organismal Systems > Immune system > Th17 cell differentiation. (View pathway)
· Organismal Systems > Endocrine system > Relaxin signaling pathway.
References
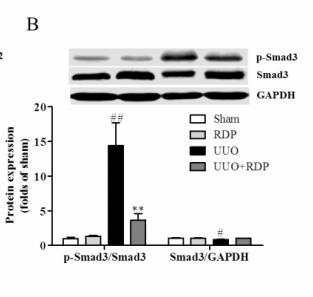
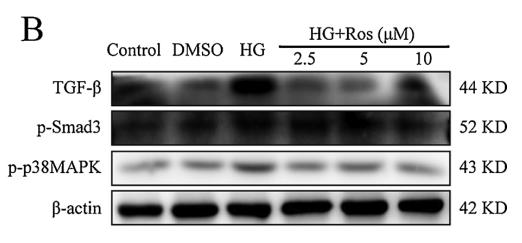
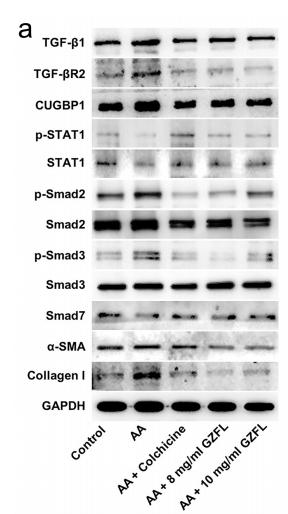
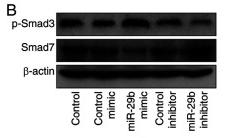
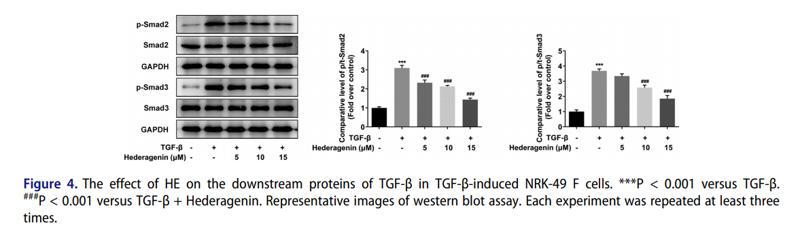
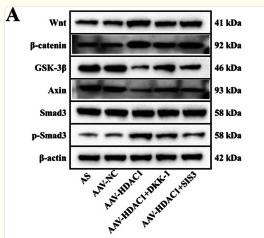
Application: WB Species: human Sample: HK2 cells
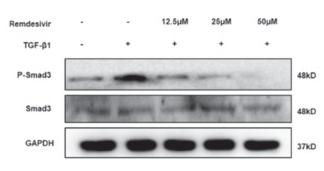
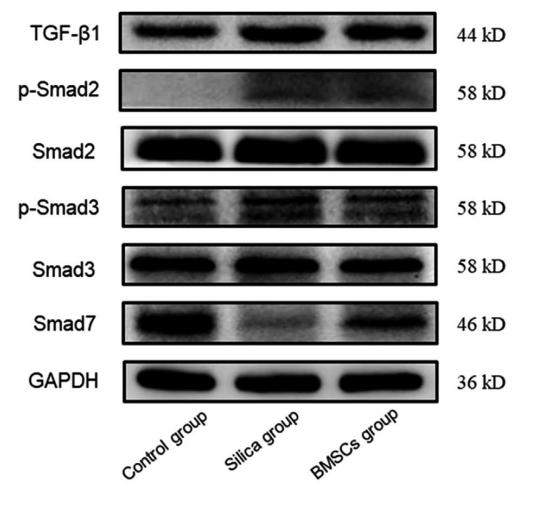
Application: WB Species: Mice Sample: H22 cells
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.