Cyclin B1 Antibody - #AF6168
| Product: | Cyclin B1 Antibody |
| Catalog: | AF6168 |
| Description: | Rabbit polyclonal antibody to Cyclin B1 |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Bovine, Horse, Sheep, Rabbit, Dog |
| Mol.Wt.: | 60kDa; 48kD(Calculated). |
| Uniprot: | P14635 |
| RRID: | AB_2835034 |
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF6168, RRID:AB_2835034.
Fold/Unfold
CCNB 1; CCNB; ccnb1; CCNB1_HUMAN; Cyclin B1; G2 mitotic specific cyclin B1; G2/mitotic-specific cyclin-B1;
Immunogens
- P14635 CCNB1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MALRVTRNSKINAENKAKINMAGAKRVPTAPAATSKPGLRPRTALGDIGNKVSEQLQAKMPMKKEAKPSATGKVIDKKLPKPLEKVPMLVPVPVSEPVPEPEPEPEPEPVKEEKLSPEPILVDTASPSPMETSGCAPAEEDLCQAFSDVILAVNDVDAEDGADPNLCSEYVKDIYAYLRQLEEEQAVRPKYLLGREVTGNMRAILIDWLVQVQMKFRLLQETMYMTVSIIDRFMQNNCVPKKMLQLVGVTAMFIASKYEEMYPPEIGDFAFVTDNTYTKHQIRQMEMKILRALNFGLGRPLPLHFLRRASKIGEVDVEQHTLAKYLMELTMLDYDMVHFPPSQIAAGAFCLALKILDNGEWTPTLQHYLSYTEESLLPVMQHLAKNVVMVNQGLTKHMTVKNKYATSKHAKISTLPQLNSALVQDLAKAVAKV
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
PTMs - P14635 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| T6 | Phosphorylation | Uniprot | |
| S9 | Phosphorylation | Uniprot | |
| K25 | Acetylation | Uniprot | |
| K25 | Ubiquitination | Uniprot | |
| S35 | Phosphorylation | Uniprot | |
| K36 | Ubiquitination | Uniprot | |
| K51 | Ubiquitination | Uniprot | |
| S69 | Phosphorylation | Uniprot | |
| K73 | Acetylation | Uniprot | |
| S95 | Phosphorylation | Uniprot | |
| K111 | Sumoylation | Uniprot | |
| S116 | Phosphorylation | Uniprot | |
| S126 | Phosphorylation | P06493 (CDK1) , P28482 (MAPK1) , P53350 (PLK1) | Uniprot |
| S128 | Phosphorylation | P06493 (CDK1) , P53350 (PLK1) , P28482 (MAPK1) | Uniprot |
| S133 | Phosphorylation | P53350 (PLK1) , Q9H4B4 (PLK3) | Uniprot |
| S147 | Phosphorylation | P53350 (PLK1) | Uniprot |
| Y177 | Phosphorylation | Uniprot | |
| K190 | Ubiquitination | Uniprot | |
| K279 | Ubiquitination | Uniprot | |
| K311 | Ubiquitination | Uniprot | |
| T321 | Phosphorylation | Uniprot | |
| T362 | Phosphorylation | Uniprot | |
| T395 | Phosphorylation | Uniprot | |
| K396 | Ubiquitination | Uniprot | |
| K411 | Ubiquitination | Uniprot | |
| S413 | Phosphorylation | Uniprot | |
| K428 | Ubiquitination | Uniprot |
PTMs - P14635 As Enzyme
Research Backgrounds
Essential for the control of the cell cycle at the G2/M (mitosis) transition.
Ubiquitinated by the SCF(NIPA) complex during interphase, leading to its destruction. Not ubiquitinated during G2/M phases.
Phosphorylated by PLK1 at Ser-133 on centrosomes during prophase: phosphorylation by PLK1 does not cause nuclear import. Phosphorylation at Ser-147 was also reported to be mediated by PLK1 but Ser-133 seems to be the primary phosphorylation site.
Cytoplasm. Nucleus. Cytoplasm>Cytoskeleton>Microtubule organizing center>Centrosome.
Interacts with the CDC2 protein kinase to form a serine/threonine kinase holoenzyme complex also known as maturation promoting factor (MPF). The cyclin subunit imparts substrate specificity to the complex. Binds HEI10. Interacts with catalytically active RALBP1 and CDC2 during mitosis to form an endocytotic complex during interphase. Interacts with CCNF; interaction is required for nuclear localization. Interacts with CDK5RAP3. Interacts with RFPL4A and UBE2A (By similarity). Interacts with INCA1.
Belongs to the cyclin family. Cyclin AB subfamily.
Research Fields
· Cellular Processes > Cell growth and death > Cell cycle. (View pathway)
· Cellular Processes > Cell growth and death > Oocyte meiosis. (View pathway)
· Cellular Processes > Cell growth and death > p53 signaling pathway. (View pathway)
· Cellular Processes > Cell growth and death > Cellular senescence. (View pathway)
· Environmental Information Processing > Signal transduction > FoxO signaling pathway. (View pathway)
· Organismal Systems > Endocrine system > Progesterone-mediated oocyte maturation.
References
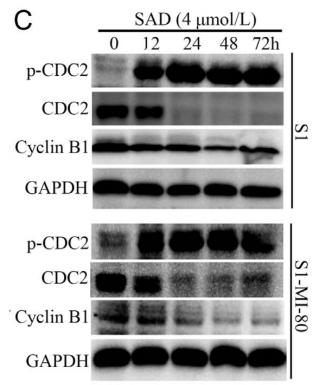
Application: WB Species: human Sample: S1 and S1-MI-80 cells
Application: WB Species: human Sample: U251 glioma cells
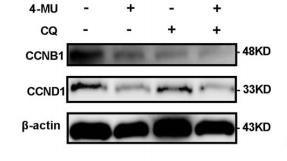
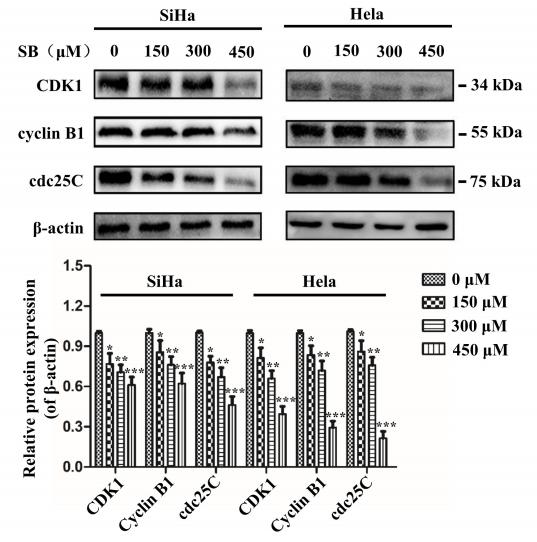
Application: WB Species: human Sample: cervical cancer cells
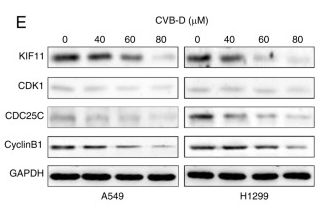
Application: WB Species: Human Sample: NSCLC cells
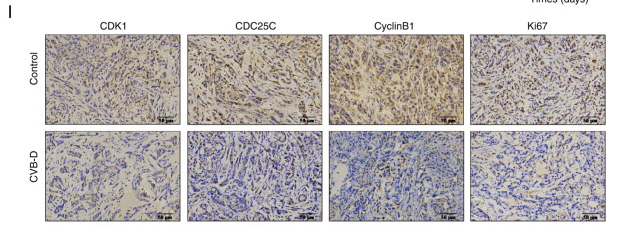
Application: IHC Species: Mouse Sample: A549 cells
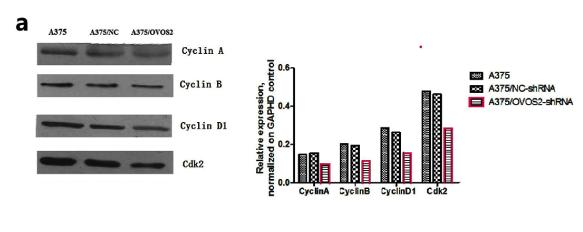
Application: WB Species: mouse Sample: A375 cells
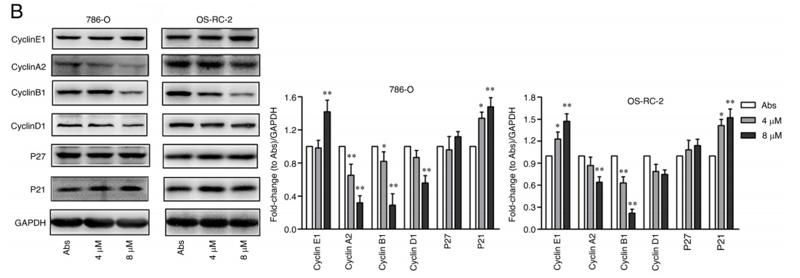
Application: WB Species: human Sample: 786‑O and OS‑RC‑2 cells
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.