DR4 Antibody - #AF0304
| Product: | DR4 Antibody |
| Catalog: | AF0304 |
| Description: | Rabbit polyclonal antibody to DR4 |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Mol.Wt.: | 50kDa; 50kD(Calculated). |
| Uniprot: | O00220 |
| RRID: | AB_2833468 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF0304, RRID:AB_2833468.
Fold/Unfold
Apo2; CD261; Cytotoxic TRAIL receptor; Death receptor 4; DR4; NF related apoptosis-inducing ligand receptor 1; TNF receptor superfamily member 10a; TNF-related apoptosis-inducing ligand receptor 1; TNFRSF10A; TR10A_HUMAN; TRAIL receptor 1; TRAIL-R1; TRAILR 1; TRAILR1; Tumor necrosis factor receptor superfamily member 10A; Tumor necrosis factor receptor superfamily member 10a variant 2; Tumor necrosis factor receptor superfamily, member 10a;
Immunogens
Widely expressed. High levels are found in spleen, peripheral blood leukocytes, small intestine and thymus, but also in K-562 erythroleukemia cells, MCF-7 breast carcinoma cells and activated T-cells.
- O00220 TR10A_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MAPPPARVHLGAFLAVTPNPGSAASGTEAAAATPSKVWGSSAGRIEPRGGGRGALPTSMGQHGPSARARAGRAPGPRPAREASPRLRVHKTFKFVVVGVLLQVVPSSAATIKLHDQSIGTQQWEHSPLGELCPPGSHRSEHPGACNRCTEGVGYTNASNNLFACLPCTACKSDEEERSPCTTTRNTACQCKPGTFRNDNSAEMCRKCSRGCPRGMVKVKDCTPWSDIECVHKESGNGHNIWVILVVTLVVPLLLVAVLIVCCCIGSGCGGDPKCMDRVCFWRLGLLRGPGAEDNAHNEILSNADSLSTFVSEQQMESQEPADLTGVTVQSPGEAQCLLGPAEAEGSQRRRLLVPANGADPTETLMLFFDKFANIVPFDSWDQLMRQLDLTKNEIDVVRAGTAGPGDALYAMLMKWVNKTGRNASIHTLLDALERMEERHAREKIQDLLVDSGKFIYLEDGTGSAVSLE
PTMs - O00220 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| K36 | Ubiquitination | Uniprot | |
| R52 | Methylation | Uniprot | |
| S83 | Phosphorylation | Uniprot | |
| S178 | Phosphorylation | Uniprot | |
| K219 | Ubiquitination | Uniprot | |
| K273 | Ubiquitination | Uniprot | |
| C336 | S-Nitrosylation | Uniprot | |
| K391 | Ubiquitination | Uniprot | |
| S424 | Phosphorylation | Uniprot | |
| K443 | Ubiquitination | Uniprot | |
| K453 | Ubiquitination | Uniprot | |
| Y456 | Phosphorylation | Uniprot | |
| T461 | Phosphorylation | Uniprot | |
| S463 | Phosphorylation | Uniprot | |
| S466 | Phosphorylation | Uniprot |
Research Backgrounds
Receptor for the cytotoxic ligand TNFSF10/TRAIL. The adapter molecule FADD recruits caspase-8 to the activated receptor. The resulting death-inducing signaling complex (DISC) performs caspase-8 proteolytic activation which initiates the subsequent cascade of caspases (aspartate-specific cysteine proteases) mediating apoptosis. Promotes the activation of NF-kappa-B.
Membrane>Single-pass type I membrane protein.
Widely expressed. High levels are found in spleen, peripheral blood leukocytes, small intestine and thymus, but also in K-562 erythroleukemia cells, MCF-7 breast carcinoma cells and activated T-cells.
Monomer. Three TNFRSF10A molecules interact with the TNFSF10 homotrimer. Can interact with TRADD and RIPK1. Interacts with ARAP1. Interacts with HCMV protein UL141; this interaction prevents TNFRSF10A cell surface expression. In the absence of stimulation, interacts with BIRC2, DDX3X and GSK3B. The interaction with BIRC2 and DDX3X is further enhanced upon receptor stimulation and accompanied by DDX3X and BIRC2 cleavage.
Research Fields
· Cellular Processes > Cell growth and death > Apoptosis. (View pathway)
· Cellular Processes > Cell growth and death > Necroptosis. (View pathway)
· Environmental Information Processing > Signaling molecules and interaction > Cytokine-cytokine receptor interaction. (View pathway)
· Human Diseases > Infectious diseases: Viral > Measles.
· Human Diseases > Infectious diseases: Viral > Influenza A.
· Organismal Systems > Immune system > Natural killer cell mediated cytotoxicity. (View pathway)
References
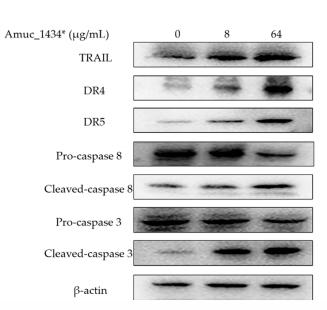
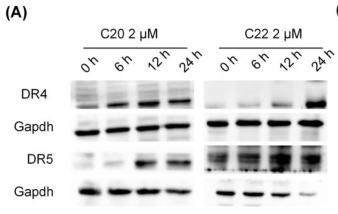
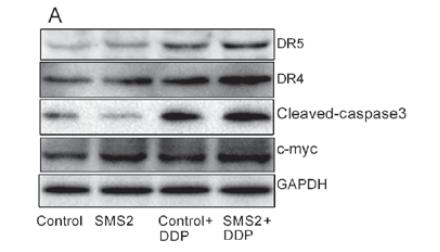
Application: WB Species: Human Sample: BEAS-2B cells
Application: WB Species: human Sample: LS174T cells
Application: WB Species: Human Sample: HepG2 cells
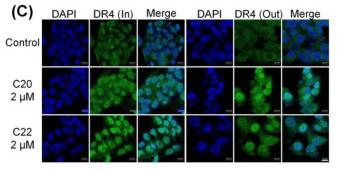
Application: IF/ICC Species: Human Sample: HepG2 cells
Application: WB Species: human Sample: HepG2 cells
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.