PRNP Antibody - #DF7034
| Product: | PRNP Antibody |
| Catalog: | DF7034 |
| Description: | Rabbit polyclonal antibody to PRNP |
| Application: | WB IHC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog |
| Mol.Wt.: | 27kDa, 40 kDa; 28kD(Calculated). |
| Uniprot: | P04156 |
| RRID: | AB_2838990 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF7034, RRID:AB_2838990.
Fold/Unfold
Alternative prion protein; major prion protein; AltPrP; ASCR; CD230; CD230 antigen; CJD; GSS; KURU; Major prion protein; p27 30; PRIO_HUMAN; Prion protein; Prion related protein; PRIP; PRNP; PrP; PrP27 30; PrP27-30; PrP33-35C; PrPC; PrPSc; Sinc;
Immunogens
- P04156 PRIO_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MANLGCWMLVLFVATWSDLGLCKKRPKPGGWNTGGSRYPGQGSPGGNRYPPQGGGGWGQPHGGGWGQPHGGGWGQPHGGGWGQPHGGGWGQGGGTHSQWNKPSKPKTNMKHMAGAAAAGAVVGGLGGYMLGSAMSRPIIHFGSDYEDRYYRENMHRYPNQVYYRPMDEYSNQNNFVHDCVNITIKQHTVTTTTKGENFTETDVKMMERVVEQMCITQYERESQAYYQRGSSMVLFSSPPVILLISFLIFLIVG
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
PTMs - P04156 As Substrate
Research Backgrounds
Its primary physiological function is unclear. May play a role in neuronal development and synaptic plasticity. May be required for neuronal myelin sheath maintenance. May promote myelin homeostasis through acting as an agonist for ADGRG6 receptor. May play a role in iron uptake and iron homeostasis. Soluble oligomers are toxic to cultured neuroblastoma cells and induce apoptosis (in vitro) (By similarity). Association with GPC1 (via its heparan sulfate chains) targets PRNP to lipid rafts. Also provides Cu(2+) or ZN(2+) for the ascorbate-mediated GPC1 deaminase degradation of its heparan sulfate side chains (By similarity).
The glycosylation pattern (the amount of mono-, di- and non-glycosylated forms or glycoforms) seems to differ in normal and CJD prion.
Cell membrane>Lipid-anchor. Golgi apparatus.
Note: Targeted to lipid rafts via association with the heparan sulfate chains of GPC1. Colocates, in the presence of Cu(2+), to vesicles in para- and perinuclear regions, where both proteins undergo internalization. Heparin displaces PRNP from lipid rafts and promotes endocytosis.
Monomer and homodimer. Has a tendency to aggregate into amyloid fibrils containing a cross-beta spine, formed by a steric zipper of superposed beta-strands. Soluble oligomers may represent an intermediate stage on the path to fibril formation. Copper binding may promote oligomerization. Interacts with GRB2, APP, ERI3/PRNPIP and SYN1. Mislocalized cytosolically exposed PrP interacts with MGRN1; this interaction alters MGRN1 subcellular location and causes lysosomal enlargement (By similarity). Interacts with KIAA1191. Interacts with ADGRG6 (By similarity).
The normal, monomeric form, PRPN(C), has a mainly alpha-helical structure. Misfolding of this form produces a disease-associated, protease-resistant form, PRPN (Sc), accompanied by a large increase of the beta-sheet content and formation of amyloid fibrils. These fibrils consist of a cross-beta spine, formed by a steric zipper of superposed beta-strands. Disease mutations may favor intermolecular contacts via short beta strands, and may thereby trigger oligomerization. In addition, the heparan-sulfate proteoglycan, GPC1, promotes the association of PRPN (C) to lipid rafts and appears to facilitate the conversion to PRPN (Sc).
Contains an N-terminal region composed of octamer repeats. At low copper concentrations, the sidechains of His residues from three or four repeats contribute to the binding of a single copper ion. Alternatively, a copper ion can be bound by interaction with the sidechain and backbone amide nitrogen of a single His residue. The observed copper binding stoichiometry suggests that two repeat regions cooperate to stabilize the binding of a single copper ion. At higher copper concentrations, each octamer can bind one copper ion by interactions with the His sidechain and Gly backbone atoms. A mixture of binding types may occur, especially in the case of octamer repeat expansion. Copper binding may stabilize the conformation of this region and may promote oligomerization.
Belongs to the prion family.
Research Fields
· Cellular Processes > Cell growth and death > Ferroptosis. (View pathway)
· Human Diseases > Neurodegenerative diseases > Prion diseases.
References
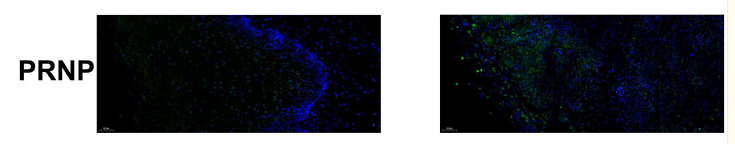
Application: IF/ICC Species: Human Sample: ESCC
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.