Tubulin beta Antibody - #AF7011
| Product: | Tubulin beta Antibody |
| Catalog: | AF7011 |
| Description: | Rabbit polyclonal antibody to Tubulin beta |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Mol.Wt.: | 55kDa; 50kD(Calculated). |
| Uniprot: | P07437 |
| RRID: | AB_2827688 |
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF7011, RRID:AB_2827688.
Fold/Unfold
TUBB3, CDCBM, Beta III Tubulin, Class III beta-tubulin, TUBB4, Tubulin, beta 3, Tubulin beta-III, Tubulin beta-3 chain, Tubulin beta-4 chain, Tubulin, beta 3 class III, CFEOM3A
Immunogens
A synthesized peptide derived from human Tubulin beta.
- P07437 TBB5_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MREIVHIQAGQCGNQIGAKFWEVISDEHGIDPTGTYHGDSDLQLDRISVYYNEATGGKYVPRAILVDLEPGTMDSVRSGPFGQIFRPDNFVFGQSGAGNNWAKGHYTEGAELVDSVLDVVRKEAESCDCLQGFQLTHSLGGGTGSGMGTLLISKIREEYPDRIMNTFSVVPSPKVSDTVVEPYNATLSVHQLVENTDETYCIDNEALYDICFRTLKLTTPTYGDLNHLVSATMSGVTTCLRFPGQLNADLRKLAVNMVPFPRLHFFMPGFAPLTSRGSQQYRALTVPELTQQVFDAKNMMAACDPRHGRYLTVAAVFRGRMSMKEVDEQMLNVQNKNSSYFVEWIPNNVKTAVCDIPPRGLKMAVTFIGNSTAIQELFKRISEQFTAMFRRKAFLHWYTGEGMDEMEFTEAESNMNDLVSEYQQYQDATAEEEEDFGEEAEEEA
PTMs - P07437 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| R2 | Methylation | Uniprot | |
| C12 | S-Nitrosylation | Uniprot | |
| K19 | Acetylation | Uniprot | |
| K19 | Methylation | Uniprot | |
| K19 | Ubiquitination | Uniprot | |
| S25 | Phosphorylation | Uniprot | |
| T33 | Phosphorylation | Uniprot | |
| T35 | Phosphorylation | Uniprot | |
| Y36 | Phosphorylation | Uniprot | |
| S40 | Phosphorylation | Uniprot | |
| R46 | Methylation | Uniprot | |
| S48 | Phosphorylation | Uniprot | |
| Y50 | Phosphorylation | Uniprot | |
| Y51 | Phosphorylation | Uniprot | |
| T55 | Phosphorylation | Uniprot | |
| K58 | Acetylation | Uniprot | |
| K58 | Methylation | Uniprot | |
| K58 | Sumoylation | Uniprot | |
| K58 | Ubiquitination | Uniprot | |
| Y59 | Phosphorylation | Uniprot | |
| T72 | Phosphorylation | Uniprot | |
| S75 | Phosphorylation | Uniprot | |
| S78 | Phosphorylation | Uniprot | |
| S95 | Phosphorylation | Uniprot | |
| K103 | Acetylation | Uniprot | |
| K103 | Sumoylation | Uniprot | |
| K103 | Ubiquitination | Uniprot | |
| Y106 | Phosphorylation | Uniprot | |
| T107 | Phosphorylation | Uniprot | |
| S115 | Phosphorylation | Uniprot | |
| K122 | Ubiquitination | Uniprot | |
| S126 | Phosphorylation | Uniprot | |
| T136 | Phosphorylation | Uniprot | |
| S138 | Phosphorylation | Uniprot | |
| T143 | Phosphorylation | Uniprot | |
| S145 | Phosphorylation | Uniprot | |
| T149 | Phosphorylation | Uniprot | |
| S153 | Phosphorylation | Uniprot | |
| K154 | Ubiquitination | Uniprot | |
| Y159 | Phosphorylation | Uniprot | |
| R162 | Methylation | Uniprot | |
| T166 | Phosphorylation | Uniprot | |
| S168 | Phosphorylation | Uniprot | |
| S172 | Phosphorylation | Uniprot | |
| K174 | Ubiquitination | Uniprot | |
| Y183 | Phosphorylation | Uniprot | |
| T196 | Phosphorylation | Uniprot | |
| T199 | Phosphorylation | Uniprot | |
| Y200 | Phosphorylation | Uniprot | |
| Y208 | Phosphorylation | Uniprot | |
| K216 | Ubiquitination | Uniprot | |
| T218 | Phosphorylation | Uniprot | |
| T219 | Phosphorylation | Uniprot | |
| T221 | Phosphorylation | Uniprot | |
| Y222 | Phosphorylation | Uniprot | |
| S234 | Phosphorylation | Uniprot | |
| C239 | S-Nitrosylation | Uniprot | |
| K252 | Sumoylation | Uniprot | |
| K252 | Ubiquitination | Uniprot | |
| T274 | Phosphorylation | Uniprot | |
| S275 | Phosphorylation | Q5TCY1 (TTBK1) | Uniprot |
| R276 | Methylation | Uniprot | |
| S278 | Phosphorylation | Uniprot | |
| Y281 | Phosphorylation | Uniprot | |
| T285 | Phosphorylation | Uniprot | |
| T290 | Phosphorylation | Uniprot | |
| K297 | Ubiquitination | Uniprot | |
| C303 | S-Nitrosylation | Uniprot | |
| Y310 | Phosphorylation | Uniprot | |
| T312 | Phosphorylation | Uniprot | |
| R318 | Methylation | Uniprot | |
| S322 | Phosphorylation | Uniprot | |
| K324 | Acetylation | Uniprot | |
| K324 | Sumoylation | Uniprot | |
| K324 | Ubiquitination | Uniprot | |
| K336 | Acetylation | Uniprot | |
| K336 | Ubiquitination | Uniprot | |
| S338 | Phosphorylation | Uniprot | |
| S339 | Phosphorylation | Uniprot | |
| Y340 | Phosphorylation | Uniprot | |
| K350 | Sumoylation | Uniprot | |
| K350 | Ubiquitination | Uniprot | |
| T351 | Phosphorylation | Uniprot | |
| K362 | Ubiquitination | Uniprot | |
| T366 | Phosphorylation | Uniprot | |
| S371 | Phosphorylation | Uniprot | |
| K379 | Acetylation | Uniprot | |
| K379 | Ubiquitination | Uniprot | |
| S382 | Phosphorylation | Uniprot | |
| T386 | Phosphorylation | Uniprot | |
| K392 | Ubiquitination | Uniprot | |
| T399 | Phosphorylation | Uniprot | |
| Y422 | Phosphorylation | Uniprot | |
| Y425 | Phosphorylation | Uniprot | |
| T429 | Phosphorylation | Uniprot |
Research Backgrounds
Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain.
Some glutamate residues at the C-terminus are polyglutamylated, resulting in polyglutamate chains on the gamma-carboxyl group. Polyglutamylation plays a key role in microtubule severing by spastin (SPAST). SPAST preferentially recognizes and acts on microtubules decorated with short polyglutamate tails: severing activity by SPAST increases as the number of glutamates per tubulin rises from one to eight, but decreases beyond this glutamylation threshold.
Some glutamate residues at the C-terminus are monoglycylated but not polyglycylated due to the absence of functional TTLL10 in human. Monoglycylation is mainly limited to tubulin incorporated into axonemes (cilia and flagella). Both polyglutamylation and monoglycylation can coexist on the same protein on adjacent residues, and lowering glycylation levels increases polyglutamylation, and reciprocally. The precise function of monoglycylation is still unclear (Probable).
Phosphorylated on Ser-172 by CDK1 during the cell cycle, from metaphase to telophase, but not in interphase. This phosphorylation inhibits tubulin incorporation into microtubules.
Cytoplasm>Cytoskeleton.
Ubiquitously expressed with highest levels in spleen, thymus and immature brain.
Heterodimer of alpha and beta chains. A typical microtubule is a hollow water-filled tube with an outer diameter of 25 nm and an inner diameter of 15 nM. Alpha-beta heterodimers associate head-to-tail to form protofilaments running lengthwise along the microtubule wall with the beta-tubulin subunit facing the microtubule plus end conferring a structural polarity. Microtubules usually have 13 protofilaments but different protofilament numbers can be found in some organisms and specialized cells. Interacts with PIFO. Interacts with DIAPH1. Interacts with MX1 (By similarity). May interact with RNABP10 (By similarity). Interacts with CFAP157 (By similarity).
The highly acidic C-terminal region may bind cations such as calcium.
Belongs to the tubulin family.
Research Fields
· Cellular Processes > Transport and catabolism > Phagosome. (View pathway)
· Cellular Processes > Cellular community - eukaryotes > Gap junction. (View pathway)
· Human Diseases > Infectious diseases: Bacterial > Pathogenic Escherichia coli infection.
References
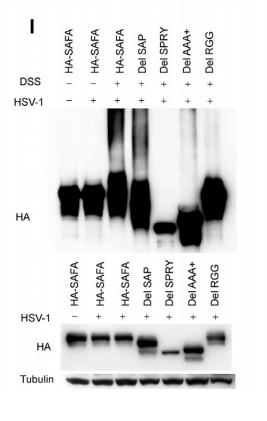
Application: WB Species: human Sample: HEK293 cells
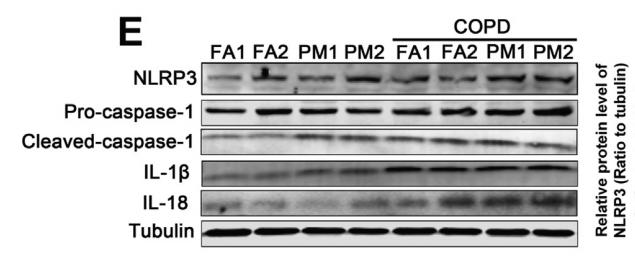
Application: WB Species: mouse Sample: lung
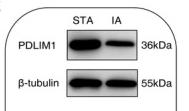
Application: WB Species: Human Sample: IA tissue
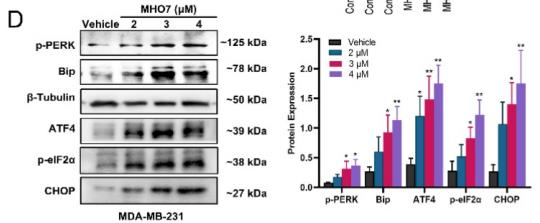
Application: WB Species: human Sample: MDA-MB-231 cells
Application: WB Species: mice Sample: cortex and hippocampus
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.