LCN2 Antibody - #DF6816
| Product: | LCN2 Antibody |
| Catalog: | DF6816 |
| Description: | Rabbit polyclonal antibody to LCN2 |
| Application: | WB IHC |
| Cited expt.: | WB, IHC |
| Reactivity: | Human, Mouse, Rat |
| Mol.Wt.: | 23kDa; 23kD(Calculated). |
| Uniprot: | P80188 |
| RRID: | AB_2838777 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF6816, RRID:AB_2838777.
Fold/Unfold
24p3; 25 kDa alpha 2 microglobulin related subunit of MMP9; 25 kDa alpha-2-microglobulin-related subunit of MMP-9; Alpha 2 microglobulin related protein; HGNC:6526; HNL; Lcn 2; Lcn2; Lipocalin-2; Migration stimulating factor inhibitor; MSFI; Neutrophil gelatinase associated lipocalin; Neutrophil gelatinase associated lipocalin precursor; Neutrophil gelatinase-associated lipocalin; NGAL; NGAL_HUMAN; Oncogene 24p3; Oncogenic lipocalin 24p3; p25; Siderocalin; siderocalin LCN2; SV40 induced 24P3 protein; Uterocalin;
Immunogens
A synthesized peptide derived from human LCN2, corresponding to a region within the internal amino acids.
Detected in neutrophils (at protein level) (PubMed:7683678, PubMed:8298140). Expressed in bone marrow and in tissues that are prone to exposure to microorganism. High expression is found in bone marrow as well as in uterus, prostate, salivary gland, stomach, appendix, colon, trachea and lung. Not found in the small intestine or peripheral blood leukocytes.
- P80188 NGAL_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MPLGLLWLGLALLGALHAQAQDSTSDLIPAPPLSKVPLQQNFQDNQFQGKWYVVGLAGNAILREDKDPQKMYATIYELKEDKSYNVTSVLFRKKKCDYWIRTFVPGCQPGEFTLGNIKSYPGLTSYLVRVVSTNYNQHAMVFFKKVSQNREYFKITLYGRTKELTSELKENFIRFSKSLGLPENHIVFPVPIDQCIDG
Research Backgrounds
Iron-trafficking protein involved in multiple processes such as apoptosis, innate immunity and renal development. Binds iron through association with 2,5-dihydroxybenzoic acid (2,5-DHBA), a siderophore that shares structural similarities with bacterial enterobactin, and delivers or removes iron from the cell, depending on the context. Iron-bound form (holo-24p3) is internalized following binding to the SLC22A17 (24p3R) receptor, leading to release of iron and subsequent increase of intracellular iron concentration. In contrast, association of the iron-free form (apo-24p3) with the SLC22A17 (24p3R) receptor is followed by association with an intracellular siderophore, iron chelation and iron transfer to the extracellular medium, thereby reducing intracellular iron concentration. Involved in apoptosis due to interleukin-3 (IL3) deprivation: iron-loaded form increases intracellular iron concentration without promoting apoptosis, while iron-free form decreases intracellular iron levels, inducing expression of the proapoptotic protein BCL2L11/BIM, resulting in apoptosis (By similarity). Involved in innate immunity; limits bacterial proliferation by sequestering iron bound to microbial siderophores, such as enterobactin. Can also bind siderophores from M.tuberculosis.
Secreted. Cytoplasmic granule lumen. Cytoplasmic vesicle lumen.
Note: Upon binding to the SLC22A17 (24p3R) receptor, it is internalized (By similarity). Releases the bound iron in the acidic lumen of cytoplasmic vesicles (PubMed:12453413, PubMed:20581821).
Detected in neutrophils (at protein level). Expressed in bone marrow and in tissues that are prone to exposure to microorganism. High expression is found in bone marrow as well as in uterus, prostate, salivary gland, stomach, appendix, colon, trachea and lung. Not found in the small intestine or peripheral blood leukocytes.
Belongs to the calycin superfamily. Lipocalin family.
Research Fields
· Organismal Systems > Immune system > IL-17 signaling pathway. (View pathway)
References
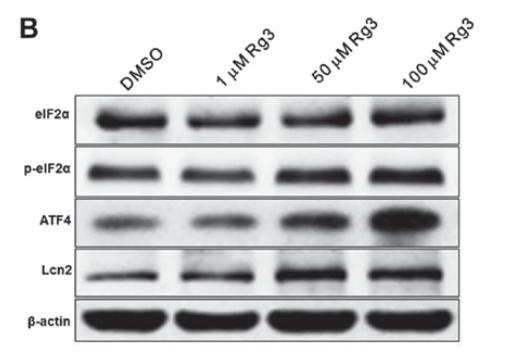
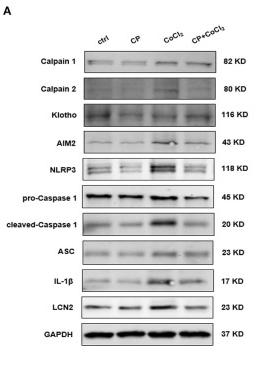
Application: WB Species: Mouse Sample:
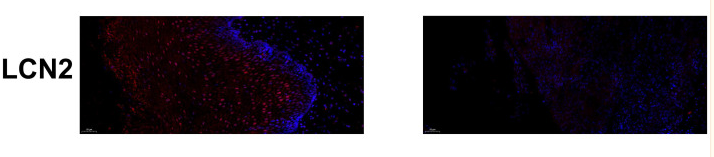
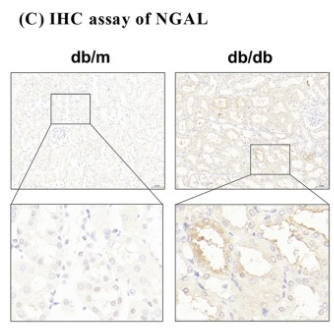
Application: IHC Species: Mouse Sample: kidney tissue
Application: WB Species: Mouse Sample: kidney tissue
Application: WB Species: Mouse Sample: GC-2 cells
Application: IHC Species: Mouse Sample: kidney
Application: WB Species: Mouse Sample: lung tissue
Application: IHC Species: Mice Sample: renal tissues
Application: WB Species: Rat Sample: kidney
Application: WB Species: Mice Sample: kidneys
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.