Collagen IV Antibody - #AF0510
| Product: | Collagen IV Antibody |
| Catalog: | AF0510 |
| Description: | Rabbit polyclonal antibody to Collagen IV |
| Application: | WB IHC IF/ICC |
| Cited expt.: | WB, IHC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog, Chicken |
| Mol.Wt.: | 160kDa,300kDa; 161kD(Calculated). |
| Uniprot: | P02462 |
| RRID: | AB_2834130 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF0510, RRID:AB_2834130.
Fold/Unfold
Arresten; BSVD; CO4A1_HUMAN; COL4A1; collagen alpha-1(IV) chain; collagen type IV alpha 1 chain; RATOR;
Immunogens
A synthesized peptide derived from human Collagen IV
- P02462 CO4A1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MGPRLSVWLLLLPAALLLHEEHSRAAAKGGCAGSGCGKCDCHGVKGQKGERGLPGLQGVIGFPGMQGPEGPQGPPGQKGDTGEPGLPGTKGTRGPPGASGYPGNPGLPGIPGQDGPPGPPGIPGCNGTKGERGPLGPPGLPGFAGNPGPPGLPGMKGDPGEILGHVPGMLLKGERGFPGIPGTPGPPGLPGLQGPVGPPGFTGPPGPPGPPGPPGEKGQMGLSFQGPKGDKGDQGVSGPPGVPGQAQVQEKGDFATKGEKGQKGEPGFQGMPGVGEKGEPGKPGPRGKPGKDGDKGEKGSPGFPGEPGYPGLIGRQGPQGEKGEAGPPGPPGIVIGTGPLGEKGERGYPGTPGPRGEPGPKGFPGLPGQPGPPGLPVPGQAGAPGFPGERGEKGDRGFPGTSLPGPSGRDGLPGPPGSPGPPGQPGYTNGIVECQPGPPGDQGPPGIPGQPGFIGEIGEKGQKGESCLICDIDGYRGPPGPQGPPGEIGFPGQPGAKGDRGLPGRDGVAGVPGPQGTPGLIGQPGAKGEPGEFYFDLRLKGDKGDPGFPGQPGMPGRAGSPGRDGHPGLPGPKGSPGSVGLKGERGPPGGVGFPGSRGDTGPPGPPGYGPAGPIGDKGQAGFPGGPGSPGLPGPKGEPGKIVPLPGPPGAEGLPGSPGFPGPQGDRGFPGTPGRPGLPGEKGAVGQPGIGFPGPPGPKGVDGLPGDMGPPGTPGRPGFNGLPGNPGVQGQKGEPGVGLPGLKGLPGLPGIPGTPGEKGSIGVPGVPGEHGAIGPPGLQGIRGEPGPPGLPGSVGSPGVPGIGPPGARGPPGGQGPPGLSGPPGIKGEKGFPGFPGLDMPGPKGDKGAQGLPGITGQSGLPGLPGQQGAPGIPGFPGSKGEMGVMGTPGQPGSPGPVGAPGLPGEKGDHGFPGSSGPRGDPGLKGDKGDVGLPGKPGSMDKVDMGSMKGQKGDQGEKGQIGPIGEKGSRGDPGTPGVPGKDGQAGQPGQPGPKGDPGISGTPGAPGLPGPKGSVGGMGLPGTPGEKGVPGIPGPQGSPGLPGDKGAKGEKGQAGPPGIGIPGLRGEKGDQGIAGFPGSPGEKGEKGSIGIPGMPGSPGLKGSPGSVGYPGSPGLPGEKGDKGLPGLDGIPGVKGEAGLPGTPGPTGPAGQKGEPGSDGIPGSAGEKGEPGLPGRGFPGFPGAKGDKGSKGEVGFPGLAGSPGIPGSKGEQGFMGPPGPQGQPGLPGSPGHATEGPKGDRGPQGQPGLPGLPGPMGPPGLPGIDGVKGDKGNPGWPGAPGVPGPKGDPGFQGMPGIGGSPGITGSKGDMGPPGVPGFQGPKGLPGLQGIKGDQGDQGVPGAKGLPGPPGPPGPYDIIKGEPGLPGPEGPPGLKGLQGLPGPKGQQGVTGLVGIPGPPGIPGFDGAPGQKGEMGPAGPTGPRGFPGPPGPDGLPGSMGPPGTPSVDHGFLVTRHSQTIDDPQCPSGTKILYHGYSLLYVQGNERAHGQDLGTAGSCLRKFSTMPFLFCNINNVCNFASRNDYSYWLSTPEPMPMSMAPITGENIRPFISRCAVCEAPAMVMAVHSQTIQIPPCPSGWSSLWIGYSFVMHTSAGAEGSGQALASPGSCLEEFRSAPFIECHGRGTCNYYANAYSFWLATIERSEMFKKPTPSTLKAGELRTHVSRCQVCMRRT
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Type IV collagen is the major structural component of glomerular basement membranes (GBM), forming a 'chicken-wire' meshwork together with laminins, proteoglycans and entactin/nidogen.
Arresten, comprising the C-terminal NC1 domain, inhibits angiogenesis and tumor formation. The C-terminal half is found to possess the anti-angiogenic activity. Specifically inhibits endothelial cell proliferation, migration and tube formation. Inhibits expression of hypoxia-inducible factor 1alpha and ERK1/2 and p38 MAPK activation. Ligand for alpha1/beta1 integrin.
Lysines at the third position of the tripeptide repeating unit (G-X-Y) are hydroxylated. The modified lysines can be O-glycosylated.
Contains 4-hydroxyproline (Probable). Prolines at the third position of the tripeptide repeating unit (G-X-Y) are hydroxylated in some or all of the chains (By similarity).
Contains 3-hydroxyproline. This modification occurs on the first proline residue in the sequence motif Gly-Pro-Hyp, where Hyp is 4-hydroxyproline.
Type IV collagens contain numerous cysteine residues which are involved in inter- and intramolecular disulfide bonding. 12 of these, located in the NC1 domain, are conserved in all known type IV collagens.
The trimeric structure of the NC1 domains is stabilized by covalent bonds (sulfilimine cross-links) between Lys and Met residues. These cross-links are important for the mechanical stability of the basement membrane (By similarity).
Proteolytic processing produces the C-terminal NC1 peptide, arresten.
Secreted>Extracellular space>Extracellular matrix>Basement membrane.
Highly expressed in placenta.
Alpha chains of type IV collagen have a non-collagenous domain (NC1) at their C-terminus, frequent interruptions of the G-X-Y repeats in the long central triple-helical domain (which may cause flexibility in the triple helix), and a short N-terminal triple-helical 7S domain.
Belongs to the type IV collagen family.
Research Fields
· Cellular Processes > Cellular community - eukaryotes > Focal adhesion. (View pathway)
· Environmental Information Processing > Signal transduction > PI3K-Akt signaling pathway. (View pathway)
· Environmental Information Processing > Signaling molecules and interaction > ECM-receptor interaction. (View pathway)
· Human Diseases > Infectious diseases: Parasitic > Amoebiasis.
· Human Diseases > Infectious diseases: Viral > Human papillomavirus infection.
· Human Diseases > Cancers: Overview > Pathways in cancer. (View pathway)
· Human Diseases > Cancers: Specific types > Small cell lung cancer. (View pathway)
· Organismal Systems > Endocrine system > Relaxin signaling pathway.
· Organismal Systems > Digestive system > Protein digestion and absorption.
References
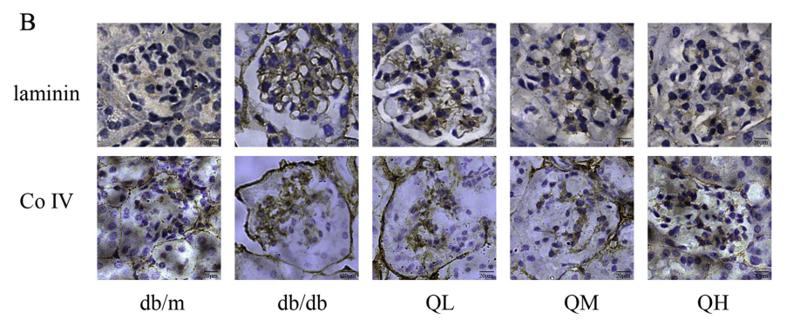
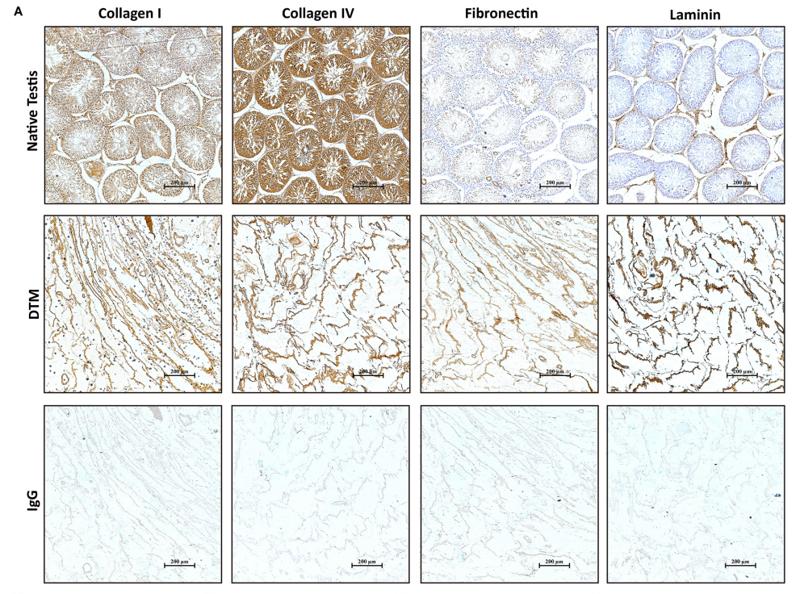
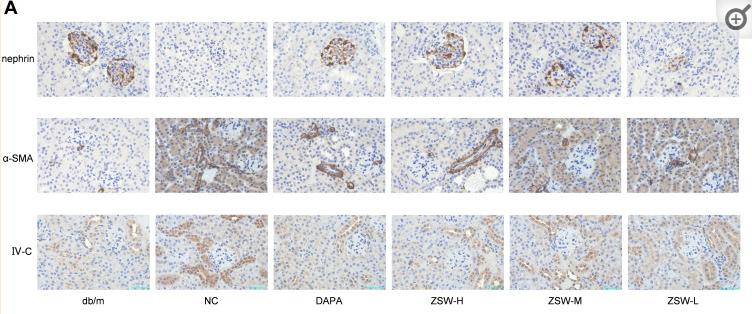
Application: IHC Species: mouse Sample: renal cortex
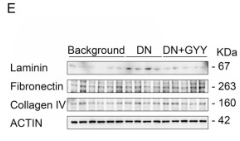
Application: WB Species: Mouse Sample: kidney tissues
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.